50 shades of biotechnology
<url>

We publish a transcript of a lecture by Alexander Panchin, popularizer of science, Candidate of Biological Sciences, senior researcher at the Institute of Information Transmission Problems of the Russian Academy of Sciences, member of the Board of the Evolution Foundation, held on February 4, 2016 in the I.S. Turgenev Library-Reading Room.
Boris Dolgin: Good evening. We are starting the next lecture of the series "Public lectures of <url>". Our guest today is a researcher at the Institute of Information Transmission Problems of the Russian Academy of Sciences, on the one hand, a professional scientist, on the other hand, an equally experienced popularizer of science in various forms: from oral, which we will get acquainted with today, to written – we see on the table a relatively recently published book "Sum of Biotechnology", Alexander Panchin. Alexander is also a member of the Board of the Evolution Foundation.
A certain number of scientists and popularizers of science, as it became clear that the Dynasty Foundation would be liquidated, decided that there should not be an empty place on the platform of informational, methodological, financial, organizational coordination of various forms of science popularization activities – and created this very foundation. Visit his website to understand how it works, to participate yourself, to understand how much you can trust the recommendations of the foundation, based on the composition of the Board. And he will be called upon to become some kind of recommendation center. You can participate in the activities of the foundation in a personal way.
But today we are not talking about the foundation, not about evolution in its natural forms, but about what is being done by human hands, artificially. Some people are very afraid of what is being done artificially. They try to find something natural all the time. Today we will just talk about the biological part of this problem.
Alexander Panchin: Thanks! It's very nice that so many people came today. To begin with, you see here paintings by famous artists painted with the help of genetically modified bacteria, in which the genes of various fluorescent proteins were introduced, they glow, maybe you could even recognize some of the paintings. This is how they look in the original.
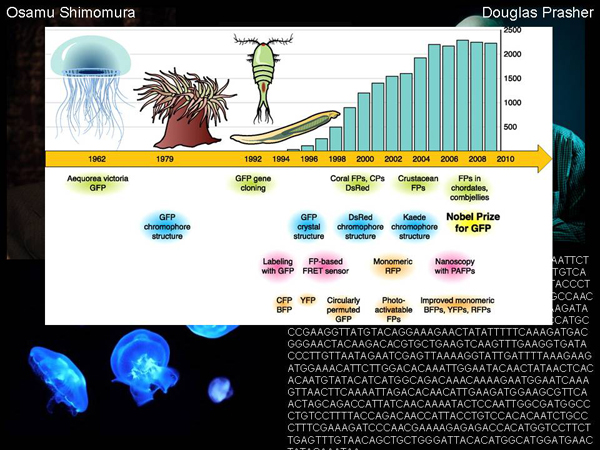
In order to draw such pictures with the help of genetically modified bacteria, scientists needed to discover a fluorescent protein gene. First, the protein itself was discovered, discovered by Osamo Shimamura, who studied glowing jellyfish, and first found a protein in them that emits blue light, carrying out a certain chemical reaction, and then found a protein that absorbs blue light and emits green.
If we look at the chronology of what happened next, we will see that since 1962 there has been a very small number of publications devoted to this very green squirrel. In general, few people were interested in this topic, except for a narrow group of scientists who learned everything about this protein: how it works, what its structure is, what is the physics of the process, how it absorbs and emits light. But it was pure fundamental science, having no applied significance.
But something happened, and the number of publications on this topic began to grow. The number of publications is growing, and in 2008 The Nobel Prize in Chemistry is given for the discovery of this protein. What happened, because of what the number of publications began to grow? What happened was that the gene of this fluorescent protein, the DNA sequence that is responsible for its synthesis, was discovered.
This sequence is depicted here. It was discovered by the scientist Douglas Prasher. As soon as this DNA sequence was discovered, it turned out that it could be transferred to other organisms and make them glow. You can transfer it to a specific genetic context to see where and in which cells certain genes work, by the glow. And such a powerful tool appeared, which is still used in molecular biology for fundamental and applied research.
Unfortunately, Douglas Prasher could not get good funding and assumed that he would not be able to continue to fully study the gene that he had discovered. And he passed this gene – literally in a test tube – to two of his colleagues, Martin Chalfie and Roger Y. Tsien, who received the Nobel Prize. Together with Osama Shimomura. Here is such a sad story – the man who discovered the DNA of a fluorescent protein did not receive the Nobel Prize.
Martin Chalfi wasn't a rich scientist either, but he was an inventive scientist. There is such a story: they needed to get a fluorescent microscope somewhere that could detect this green glow, but there was no money. And they invited microscope dealers to their laboratory, who sent their representatives with microscopes to check if everything was working. They took a microscope, quickly carried out their research, and then said that this microscope did not suit them.
And so they accumulated data for publication in the journal "Science". Here is a round worm, inside which the fluorescent protein gene works in those cells that are responsible for the body's ability to respond to physical touch. And they showed that this fluorescent protein can be transferred to other organisms, and with its help to study how and where and which genes work in the body.
And this scientist's name is Roger Tsien. In his laboratory, they took a gene of a green fluorescent protein and transferred it to various bacteria, which were then forced to multiply, and selected those bacteria that, due to some random mutations, began to glow in a different way: brighter, or in a bluer range, or in a redder range. They made a selection and with the help of such "evolution in vitro" they got bacteria with other genes of fluorescent proteins. And these are the bacteria that painted these pictures.
Martin Chalfi, Roger Tsien and Osama Shimomura received the Nobel Prize, and since more than three people are not given it, Douglas Prasher was the guest of honor at the ceremony ("Polit.ru" – Tsien and Chalfi paid for air tickets and a hotel for Prasher and his wife so that he could attend the Nobel Banquet).
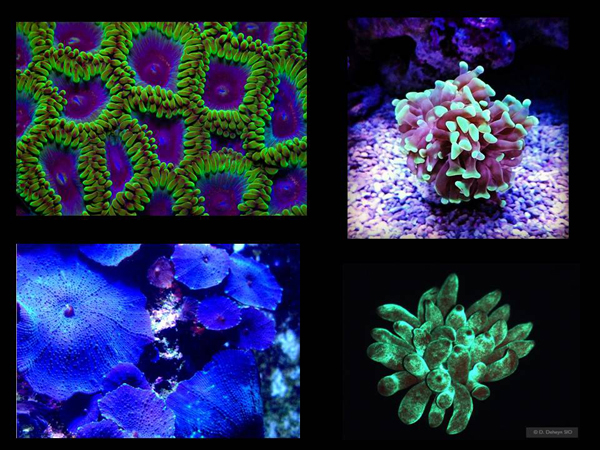
What were Russian scientists doing in the meantime? "Under the cover of night" on a yacht, with a pack of condoms and a detector of counterfeit banknotes, they performed science. Yes, oddly enough, all this was purely for scientific purposes. Although the coastal patrol had questions for them, they had everything in order with their documents. They took a counterfeit banknote detector, placed it in a condom so as not to get wet and, diving, shone ultraviolet light on various underwater life, in particular – on corals under water. And we watched which ones were glowing.
Then they took DNA samples from these organisms to find other fluorescent protein genes there. You can create new fluorescent proteins in the laboratory with the help of evolving bacteria, or you can do it by looking for ready-made solutions invented by nature in the environment.
In general, apparently, one of the main tasks of genetic engineering is to find wonderful genes that can be used further, because a lot of good things have already been invented in nature. Accordingly, what did they do next? Here one of these scientists, Mikhail Matz, breaks the structure of a green fluorescent protein, and on the left is a picture from their publication.
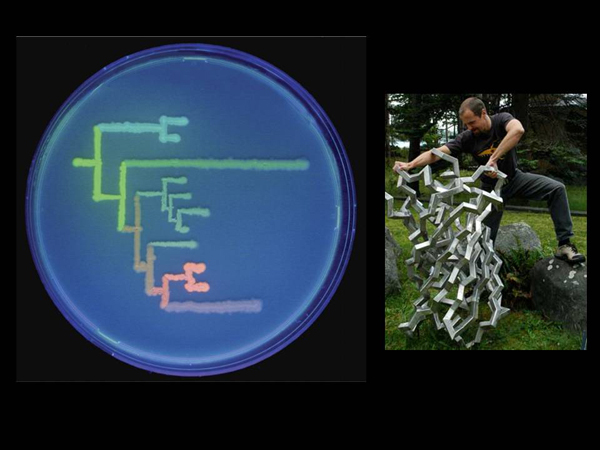
What were they doing? This is an evolutionary tree showing the relationship between some genes of fluorescent proteins. This tree is drawn by bacteria that have fluorescent protein genes. As we know from the theory of evolution, once all living organisms had a common ancestor. Similarly, once upon a time, living organisms with different fluorescent proteins once had a common ancestor who first "invented" some kind of fluorescent protein. And then his descendants modified this protein, and different colors turned out.
And so, using the methods of mathematical biology, bioinformatics, we can reconstruct this evolution, we can see what variants of these genes are now, and predict what variants were in very distant organisms that lived before and now no longer exist.
The "edges" of this tree, these "leaves", are drawn with the help of bacteria, which contain the genes of real proteins. And in the nodes there are bacteria with genes that have been reconstructed. That is, scientists took, predicted on a computer what the sequence of the gene should be, after that they synthesized it in a test tube, "inserted" it into a bacterium and looked at how it glows. By the way it glows, you can tell what color it was.
In this case – you see - there are such brownish, light green ones? These are the "ancestral" forms. Maybe there were even more ancient ones. There is such jargon in science: scientists who are engaged in "wet" biology – they drip something into test tubes, and there are "dry" biologists, like me, who sit at computers and analyze ready-made biological data. And here the approaches of real "wet" biology are combined – this man is a real wet biologist, he really dived under water for the sake of science, and dry biology to get another approach to obtaining these fluorescent proteins.
And here, in fact, the decorative value of green fluorescent protein is all sorts of beautiful little animals. There is an art section where this is used. There is such an artist – Eduardo Kac, who came up with the following thing. A certain construction has been introduced into the bacteria containing the fluorescent protein gene.
The construction looks like this: a quote from the Bible in English was taken, translated into Morse code using a certain cipher, which was translated into a sequence of nucleotides that make up the DNA molecule – A, T, G, C and so on. After that, the sequence along with the gene of the green fluorescent protein was introduced into the bacteria, and these bacteria were given time to multiply. The green fluorescent protein is just like a marker that there is this insert there.
These bacteria were given some time to mutate, and then read the DNA sequence that resulted from these mutations. And they got a new DNA sequence, which was translated into Morse code, from which they made a text. And there are mistakes in this text.
And this Eduardo Katz says that this metaphor is that a mutation in the genetic material of DNA is like a typo in the text. What can be viewed from this angle. Accordingly, typos are different. Sometimes a typo in the text leads to something insignificant: for example, instead of "Sasha rode a motorcycle" you say: "Masha rode a motorcycle." You get a text similar in meaning and, perhaps, even more correctly describing reality.
But there is all sorts of nonsense: if you make too many mutations, you can get "Porridge rode on a cotocycle". And when the body becomes ill, it has already happened a harmful mutation.
It is possible to develop this metaphor further, as I have tried to develop it. You can imagine the transfer of a gene from one organism to another as the transfer of a piece of text from one text to another. Here we will transfer a piece from "War and Peace" to "War of the Worlds". "... A large grayish round carcass, perhaps the size of a bear, slowly and laboriously climbed out of the cylinder. Leaning out into the light, she glistened like a wet belt. Two large dark eyes stared intently at me... the monster had a round head and, if I may say so, a face. Under the eyes is a mouth, the edges of which moved and trembled, drooling. The monster was breathing heavily, and his whole body was throbbing convulsively. One of its thin tentacles rested on the edge of the cylinder, the other it waved in the air… This fat young man was the illegitimate son of the famous Catherine nobleman, Count Bezukhov."
We transferred one meaningful fragment, received another meaningful fragment. Actually, that's what genetic engineering does.
It was an art, and now it is a fundamental science. Here on the slide shows a picture of such a "thing", which is called brainbow – "brain rainbow". Brain – brain, rainbow – rainbow. From above – a section of the brain of a rodent, from below – a section of the brain of a drosophila fly. Such a thing was invented: you can take and add to the DNA of a rodent a huge number of different copies of different genes of fluorescent proteins.
And then a certain technology was invented that allows you to make sure that as this organism develops, some copies of these genes "turn off", stop working. It happened in different ways, in different cells. As a result, different combinations of fluorescent protein genes are formed in different cells. And just as we get a huge palette of shades of colors by mixing red, blue and green, just as there is a huge palette of "shades" of individual cells. And we can, by looking at the nervous system, now understand how one nerve cell is connected to another, because a nerve cell has a body, there are processes, and it was very difficult in this confusing scheme to understand where these processes are, where they lead.
But now you see that from this cell the yellow process leads here. And you understand that this cell innervates this part of the body of this organism, and so on.
Now we will talk about how biotechnologies can be used to improve, in particular, the life of humans and other living organisms. Probably, everyone has heard the news: in the UK, they were allowed to conduct experiments on the creation of genetically modified human embryos, provided that they will not be implanted anywhere later.
A little earlier, there was a publication by scientists from China who had already made genetically modified embryos that were not implanted anywhere, and were made using a special technology called "CRISPR/Cas9" – this is a DNA editing method, which I will talk about later.
Actually, now the idea is developing that we can genetically modify embryos. When we learn how to do it well, maybe we can put it all into practice. What kind of technology was used by these Chinese scientists? They use some system that exists naturally in bacteria. It was discovered when trying to make a more delicious, less perishable yogurt.
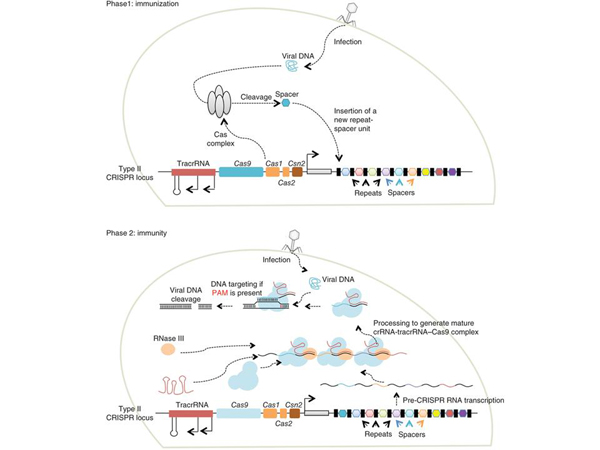
During the study of lactic acid bacteria that create yogurt, a previously unknown immune system was discovered. This system works as follows: a virus injects its DNA into a bacterial cell. Bacteria have a special mechanism that a piece of this viral DNA embeds into its DNA. Here you can see such red, blue "things" – these are the remnants of fragments of different viruses that the ancestors of this bacterium once encountered. A kind of "antivirus database" of viruses that bacteria have encountered before.
Then many copies, fragments similar to this viral "insert" are synthesized, only not DNA, but RNA. A kind of intermediate molecule between DNA and protein. In this case, no protein is produced, the RNA is recognized by a special protein that takes this RNA and uses it to start looking for other "inserts" of similar viruses. And destroy them.
Here is a viral infection, here is some mechanism that "cuts out" a piece of the virus, which is called a spacer, and this spacer is embedded in the DNA of this bacterium, that is, bacteria, in fact, are engaged in genetic engineering themselves. Here is a set of different viruses, you see – they are of different colors. And then RNA is synthesized, it binds to a protein, and this protein, like a key in a lock, tries to look for other viral sequences to find and cut them. Was it clear what I just told you? If it was unclear to someone, you can ask now.
Boris Dolgin: And this is the only exception when you can ask.
Alexander Panchin: So, what can it be used for further? Let's forget about the embryos first. Let's take plants. Bacteria for hundreds of millions of years of evolution – and maybe more, this mechanism has been debugged for a very long time – now we can take it and transfer it to another organism.
For example, in plants that do not have such an immunity mechanism. Recently, a paper was published where plants resistant to the virus were taken and made, due to the fact that this mechanism of bacterial immunity was transferred to them. It is clear that this can be used for very different purposes.
But one of the things is this: if we have learned how to make the exact place of the incision in DNA, then we can learn how to embed something very precisely there, getting various genetically modified organisms, from bacteria to humans.
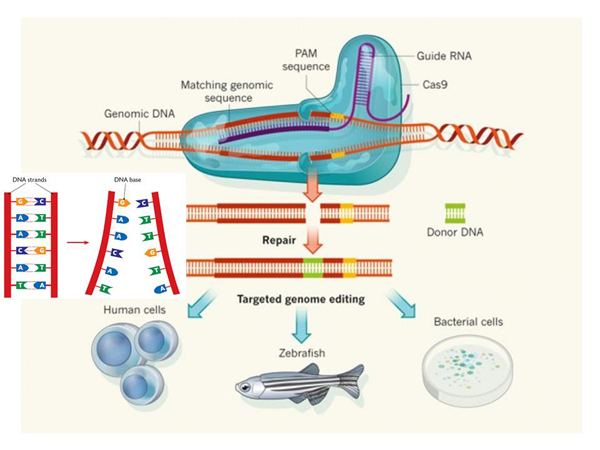
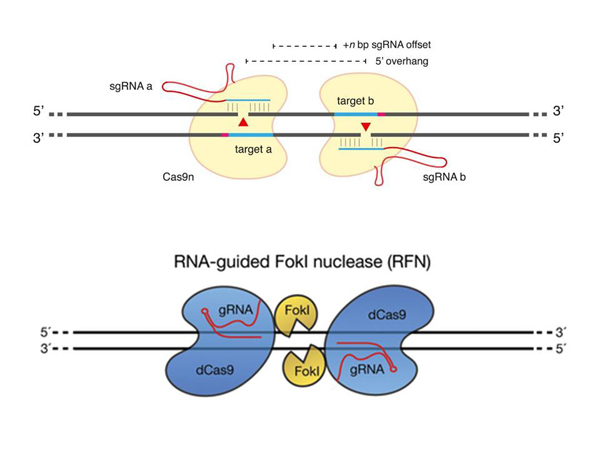
Here is a mechanism showing how the "CRISPR/Cas9" mechanism works. This red one is the DNA in which we want to make an incision. And this is the so–called "guiding RNA", the RNA of a bacterium, similar to a piece of virus that it needs to recognize. Recognition takes place here.
These teeth form complementary bonds. In a DNA molecule , complementary bonds look like this: A stands opposite T, and G stands opposite C. And the double helix of the DNA molecule is formed. And in RNA there is also a very similar thing, just instead of T – U, but it is important that complementary bonds are also formed, opposite one letter is another. And, thanks to these connections, a certain place in the DNA is recognized.
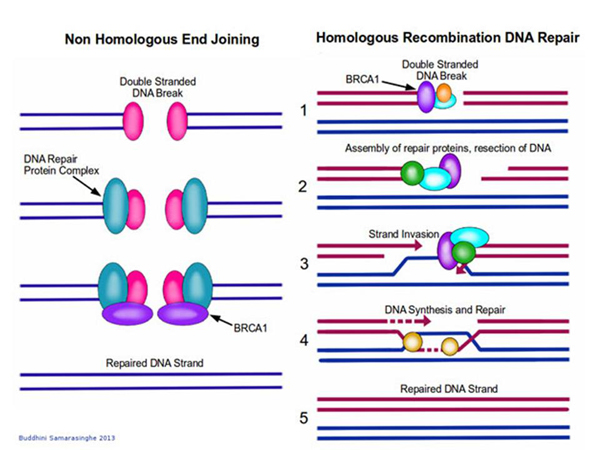
It could be some kind of genomic DNA, and this protein makes a cut here. The incision is formed, and then we want to insert something there. How? To do this, a mechanism is used that also exists in our cells, it is called the "homologous recombination mechanism".
What does it mean? Here we have DNA. Everything is the same, just a double helix turned into a chain to make it easier for you to watch. This "CRISPR/Cas9" cuts right here in the DNA molecule.
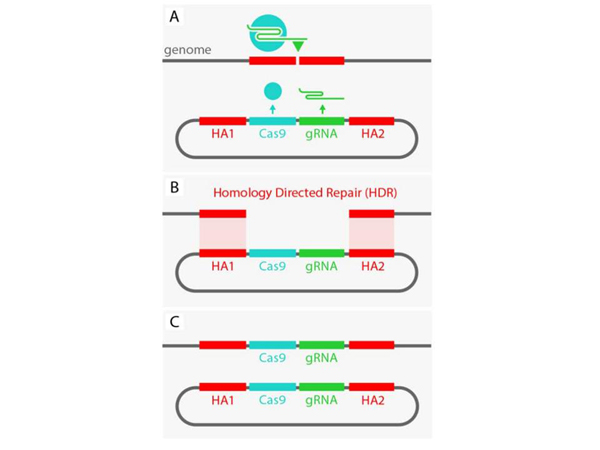
Now we still have a certain design being introduced into the cell, such a round thing – a plasmid, in which, in this case, the gene responsible for the production of the CRISPR/Cas9 protein and the production of "guiding RNA", which directs to the insertion site, is encoded. But it doesn't matter, we can insert other genes there. But it is important that this thing is framed by two sequences, they are highlighted in red. The peculiarity of each sequence is that they are complementary, that is, they form the same "teeth", let's say, with fragments that are on the sides of the insertion site. And what's going on?
The cell thinks: "Yeah, I have a "hole" in the genome, how are we going to fix it?" You can just sew it, but maybe we had something cut out? And normally, in such cases, the cell turns to the spare chromosome – how is it? If there is an insert there, then it can be copied and pasted, and the hole will be patched correctly. And in some cases, this is what happens: the cell itself builds homologous sequences opposite each other, and completes the necessary sequences.
And when Chinese scientists did their work, there was a lot of noise, there was a scandal that two leading scientific journals received an article and refused to publish it. Not because they didn't like it from a scientific point of view, but because they decided that it could be ethically wrong. However, another magazine published the article.
One of the things that Chinese scientists wrote about was that the mechanism they used was still very unreliable. Mistakes happen very often – it creates holes where it is not needed. Therefore, for the genetic engineering of plants and animals, this is all wonderful, because you can always select an organism where an insertion or incision occurred where it is needed, but it's too early to use this technology for a person, because you can accidentally get a "mutant" child who will have problems.
Therefore, let's do it on non-viable embryos for now. Since then, science has made a big step forward. I haven't had time to write about it in a book yet – when they received a mutant version of the Cas9 protein, so specific that it practically no longer makes mistakes. At least, that's what the authors of the work claim. Of course, there have been cases when the authors of the works were wrong, but this article was published in the cool journal "Nature".
In parallel with this, some very "cool" technologies have appeared, which also allow increasing the specificity of this "CRISPR/Cas9" system. One of the things that was invented is related to a mutant variant of the Cas9 protein. This is the protein. He was mutated in such a way that he could no longer make an incision in two DNA strands at the same time, only in one.
In this case, if you recognize one section of DNA, in one chain, then you have a single-stranded incision, and for the cell it's nonsense, it restores it without problems. Only the double–stranded incision presents some hitch for her - how should it be sewn back together? Therefore, they take two "guiding RNAs", two different sites that are next to each other in the DNA molecule that you want to "target", one here, the other here, and only if you have two Cas9 proteins, independently, recognizes two neighboring sites, a cut occurs in one chain and in another chain, only in this case there is a full-fledged cut, and the necessary further insertion takes place, and so on.
Another thing that was invented is even cooler. They guessed that another protein could be attached to the Cas9 protein, which also knows how to cut DNA. At the same time, the Cas9 protein itself had its ability to cut DNA canceled by mutations. That is, this Cas9 now only recognizes DNA, a certain DNA sequence that needs to be cut, but it does not cut it itself.
And in this case, the incision occurs only if one Cas9 "sat down", "recognized" a place, the other Cas9 "sat down", "recognized" a certain place, there were certain distances so that the two halves of the protein that was attached to them formed a complex, and this complex can already cut this DNA, only if there is a certain place that this protein can cut itself.
That is, there are already so many conditions for cutting that it cannot happen by chance. Thus, the accuracy of this method can be greatly improved.
With all these technologies "at hand", we are approaching a certain future, like in a game where plasmids are injected into themselves and acquire super abilities. The ability to set fire to objects has not yet been obtained, but superpower with the help of genetic engineering has already been achieved.
True, this is not related to CRISPR/Cas9, but to older technologies, but it doesn't matter. These rodents have a mutation – they have a spoiled protein that limits muscle mass. Here they are – pumped up, super-strong.
But what was done in the laboratory was done by nature 100 years ago – do you see these bulls? They were bred by breeders in the middle of the XIX century, the breed is called the Belgian blue. They are completely natural. In fact, what genetic engineering does and what happens in nature is very similar, it differs only in that mutations of all kinds occur in nature, and we need certain mutations that allow us to achieve something.
In this work, rodents were made twice as many, but there was a negative consequence – hair that grows in the wrong place. The technology has not been finalized yet, but we are striving for it. For example, there are biotechnological methods of gene therapy for adults that can overcome impotence – both in rodents and in humans.
Returning to the picture with plasmids, where they were injected into an adult, the question arises: how will we genetically modify a person?
Of course, we can genetically modify the embryo, which will turn out to be a "GMO person". But we are not experimenting on embryos yet, we want to do something good on adults for now.
It is impossible to change each of the trillions of cells that exist in humans. But it does not need to be done. Very often there are diseases associated with a change in some gene that should work in a specific type of cells, in specific conditions - for example, in liver cells. For example, hemophilia – with this disease, blood clots badly. Due to minor injuries, there is severe bleeding or severe hematomas appear.
Hemophilia is associated with a mutation of one of the blood clotting factors, which is produced by liver cells. Accordingly, we need to change not the cells of the eye, not the brain cells, not the skin cells, but the liver cells. To do this, we need a technology that can deliver a certain correct version of the gene responsible for the blood clotting factor to the liver cells, and we need a mechanism that will make this gene work there.
One of the key points here is the delivery mechanism. To do this, you can use different viruses.
Here, however, the HIV virus is shown – due to the fact that it is a virus that knows how to search for our immune system cells, so its shell is arranged. By analogy with it, other viruses are able to search for certain types of cells. There are adenoviruses that can search for liver cells. There are viruses that prefer the cells of the nervous system. There are viruses that prefer mucosal cells. Etc.
That is, you take a virus that is looking for certain cells, remove everything from it that makes it a virus, borrow only its shell. And you put the gene that needs to be delivered into it. This was done to help people suffering from hemophilia. And already now the variant of the drug for hemophilia has been clinically tested. This is already a variant of a certain solution that has not yet entered the market, but is already being tested on people with some success.
I was talking about adenoviruses – they are good because they inject their DNA into the cell nucleus, and DNA is not embedded anywhere usually, it just works. Why is it good that it is not embedded? We don't want to embed it so as not to spoil something - if something is spoiled in the DNA in an adult organism, it can lead to cancer. Here such a risk is reduced. So, adenovirus is one of the options that can be used in certain conditions.
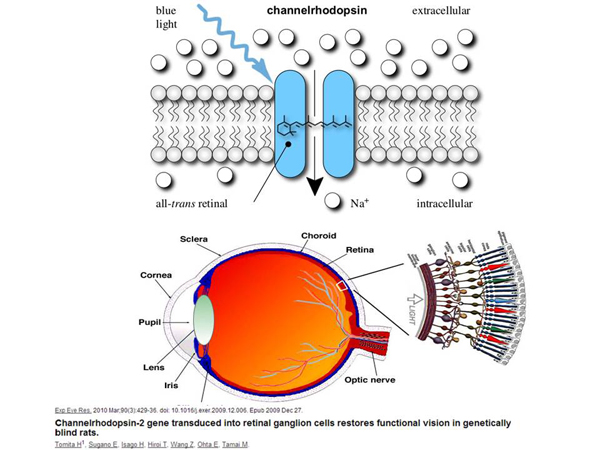
As I have already said, the task arises – to find such genes that would be good to embed. One of the most "cool" genes that can be embedded somewhere and many different technologies can be created with it is the protein kanalorodopsin, the gene of this protein. This is a protein that normally exists in algae in nature. It is located in the membrane of the algae. When blue light enters this protein, it, being a channel, opens and begins to pass through sodium ions, positively charged.
For algae, this is a signal that "there is light there", and they can somehow change their position in space in order to synthesize more efficiently. But we can take the gene of this fluorescent protein and embed it somewhere. If we embed it into the nerve cell of an animal, then, due to the fact that sodium ions are injected into the cell, the membrane potential of this cell changes.
If it were a nerve cell, it would create a nerve impulse that would be transmitted to other nerve cells. That is, we could get a nerve cell regulated by light! We shine a light on a nerve cell, and it activates. We can control the nervous system with light, almost non-invasively. Based on this, a whole science has emerged – optogenetics, which now uses this approach to study the work of the rodent brain and approaches humans.
What else can be done with this technology and the rhodopsin channel? The structure of the eye is shown here.
There is a retina in the eye, where there are light–sensitive cells that react to light - there are cones, there are rods. Cones react to green, blue and red, and sticks react to light or dark, light or dark. There are diseases in which retinal cells die. And we can – this was done on rats – make this rat see with other cells that normally should not see.
Ganglion cells are shown here, which normally do not have photosensitivity. They seem to collect a signal, but they themselves are not activated by the light. But we can introduce the rhodopsin channel gene into them, and they will be excited to blue. And now they are activated by the light themselves, they send a signal to the brain, and the brain interprets this information somehow, because it turns out that these rodents are better oriented in space than those rodents who were not subjected to gene therapy. There is a mechanism in which we can treat different variants of blindness in a very unusual way. There are other diseases that can also be treated this way, it's just not so interesting from the point of view of the mechanism.
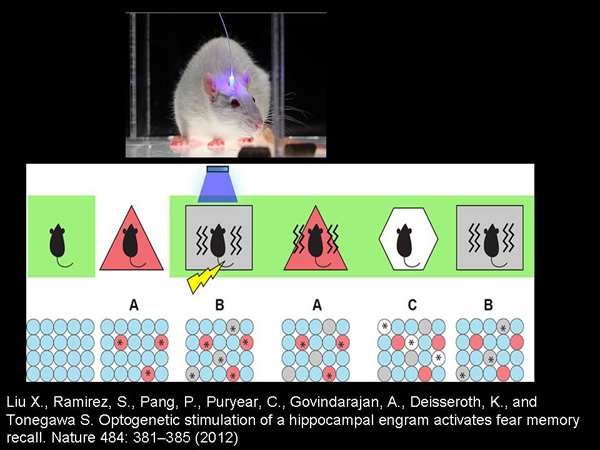
One of the most "cool" works using optogenetics is the study of Susumo Tonegawa, who showed that with the help of an optogenetic approach, it is possible to "edit" the memory of rodents. What was done? Rodents are placed in some kind of room. These are special "genetically modified" rodents whose nerve cells, if active, produce rhodopsin channel proteins and become light-activated.
And these rodents are placed in a certain room, where their nerve cells associated with the recognition and memorization of space begin to produce proteins of the rhodopsin channel. After that, the rodents are placed in another room, where they begin to be electrocuted and shone into the brain to reactivate those cells that were active in the room where they have not been electrocuted yet.
And after that, rodents begin to be afraid of the room where they were not electrocuted – only because those cells that were active when they were in that room are activated by light. It is not very clear how this should be described in terms of our perception, but the fact is that they begin to be afraid of the room where nothing bad has been done to them. This study is remarkable because it shows that memory is not a property of the eternal soul, but a material thing that we can take and fix, change. Let it be very rude for now and only on rodents.
Returning to the topic of "the other 50 shades" – these rodents are special in that they have been introduced a special gene, also associated with opto-genetics – an erectile opto-genetic stimulator, or "Eros".
When they are illuminated with blue light, they get an erection – a mechanism similar to the mechanism of action of "Viagra" is triggered. Accordingly, one can imagine in the future that a girl can give a blue flashlight to her lover – and many problems will be solved. But you can go further.
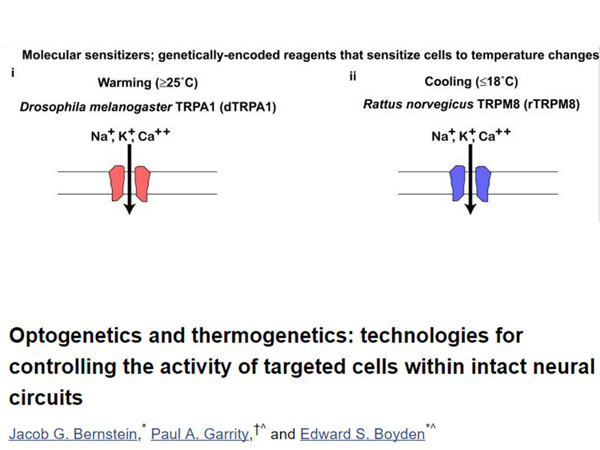
There was optogenetics, and there is also thermogenetics. Just as the rhodopsin channel protein passes sodium ions in response to light, there are proteins that pass sodium ions and other ions in response to temperature. On high temperature, on low temperature. They are very different in nature. Accordingly, you can take and make different cells – both nerve cells activated by temperature, and other cells activated by temperature.
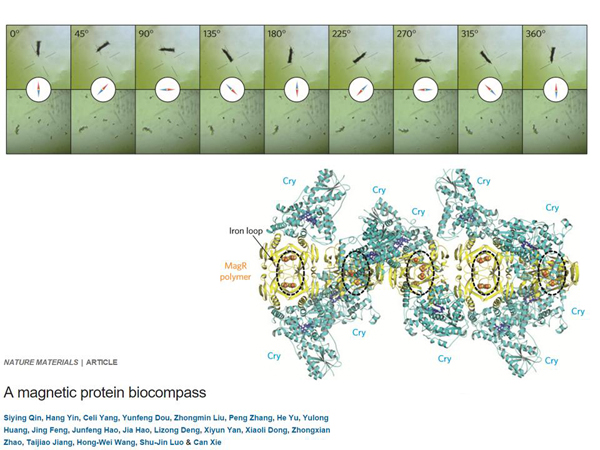
It is clear that it will be problematic to use this for the same purposes as Eros, so you can go further – magnetic genetics. It is known that some organisms have magnetic reception, for example, birds that use a magnetic field to orient themselves in space. We don't know if people have magnetic reception, but people have a protein that has it. Maybe it's a side effect of evolution. It is not known how we use it and whether we use it at all, but this picture shows aggregates of this protein – it is not one protein, it is a bunch of protein.
And you can see how it aligns with the arrow of the magnetic compass. It has a magnetic reception. He can bind pieces of iron and thereby orient himself in space.
What is remarkable about magnetic genetics? We are not yet able to make something change in the cell in response to a change in the magnetic field, but if we learn how to do this, we will be able to concentrate something locally. It will be possible to create non-invasive methods of activating some cells in response to a change in the magnetic field in the future.
Now more about diseases. Scientists have already achieved the ability to treat HIV and some types of cancer. Now I will tell you what we have achieved. One of the problems of the fight against HIV is that it is able to introduce its hereditary information into our cells, into the cells of the immune system, and there it can doze for a long time. And it seems that the virus was defeated, and then – no, there are infected cells, they begin to produce the virus. And what to do with it is not clear.
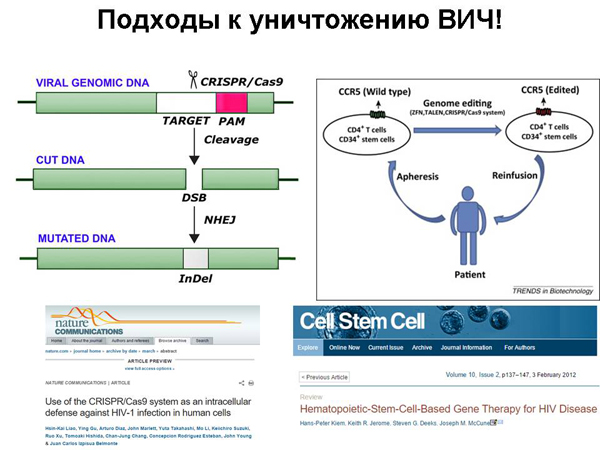
It turned out that we can learn how to cut the HIV virus from human DNA using CRISPR/Cas9 technology. We can match the guide RNA for CRISPR/Cas9 so that it matches the embedded DNA of the immunodeficiency virus. The virus itself has an RNA genome, but when it is embedded in our cells, DNA is already there. And, accordingly, CRISPR/Cas9 can cut it. And we can, firstly, make cells resistant to HIV, because they have a CRISPR/Cas9 system, which, if an "enemy" is embedded, immediately cuts it out, or we can free cells from HIV if we deliver CRISPR/Cas9 there.
So far, this has been done only on cells in culture: HIV-infected cells were obtained in a test tube, CRISPR/Cas9 was injected into them, and he cut this HIV there. There is a problem of how to fix it in a large number of cells in the body, but this technological issue will be tried in the future and will be solved someday.
Another thing that can be done against HIV is the creation of genetically modified human cells resistant to HIV. I'll tell you a wonderful story. A unique case was in Germany: a man who had two diseases at the same time – HIV and leukemia. When a person has leukemia, he is treated with special chemo and radiotherapy to kill actively dividing cells, including cancer cells. But, unfortunately, stem cells are also killed, giving rise to other cells, for example, blood cells.
A person is very ill after that, he needs a bone marrow transplant to renew these cells. In this case, we decided to use not a simple bone marrow donor, but a donor with a certain mutation. The fact is that HIV penetrates the cells of the immune system, it recognizes them – they have a special CCR5 protein on their surface, in addition to other proteins. If this protein is corrupted – and there are people who have it corrupted – then HIV cannot enter this cell.
And there are people in nature who are resistant to HIV. And in this case, we found a donor who has this mutation – with HIV-resistant cells. The patient received a bone marrow transplant from this donor, and since then, neither HIV nor leukemia have been detected in this patient.
But the problem with using this approach is that chemo- and radiotherapy with bone marrow transplantation are very dangerous procedures, if a person is sick with HIV and takes drugs against him, then his expected life time may be 30-40 years. Thanks to good antiviral drugs. If he does such an operation, then he has a fairly high chance of dying right during the operation. But, on the other hand, such a solution gets rid of HIV, apparently, forever.
So what can we do with genetic engineering technology? We can take a person, take his immune system cells, "spoil" them a little so that they are resistant to HIV, and then this person will have a population of immune system cells resistant to HIV and will not lose all his immunity.
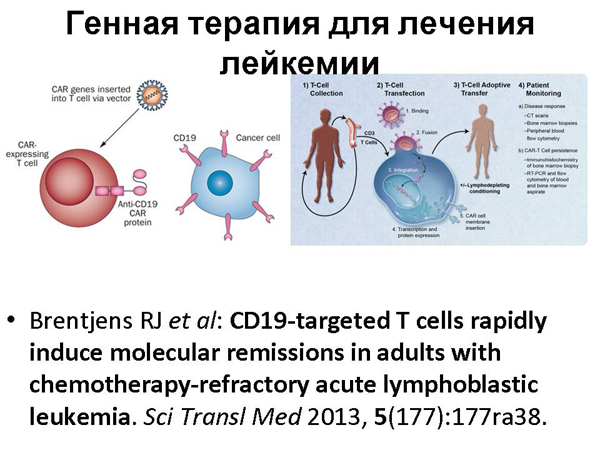
Cancer. Cancer cells are cells that have "gone crazy", in fact. Because of the mutation, they begin to divide endlessly, which causes a cancerous tumor. Our immune system is constantly trying to fight cancer cells – it is trying to find such a mutated cell and destroy it. But sometimes he misses one. But sometimes we know what the molecule that recognizes this type of cancer cell should be in order to fight it. Because there is already huge data on different types of cancer cells and in general, there is a science that describes which immune cells are fighting which.
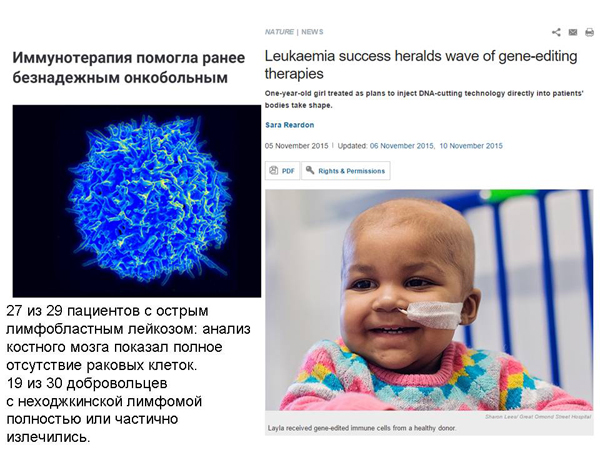
We can take cells from a patient's immune system and compare them with receptors, molecules that they use to recognize this type of cancer. This does not help in all cases of cancer, but it helps. Several people were cured in this way, however, in combination with chemotherapy, but still. And there was a story about a girl who was cured of leukemia.
When we talk about modified human immune cells, this is practically your individual medicine. It's very expensive. Scientists modify your own cells in the laboratory, it can never become an industrial, mass phenomenon. And I would like to see a cure for cancer that can be bought, roughly speaking, in a pharmacy.
And the idea arose: to make such "universal" immune cells that are not recognized by the immune system and therefore they can be given to different people, and they contain the necessary protection from both chemo and radio therapy to combine with chemo and radio therapy, and at the same time so that they can recognize cancer cells and fight them. And these ideas were tested on this girl. It is clear that clinical trials should be more than once, but, in my opinion, all this is very encouraging.
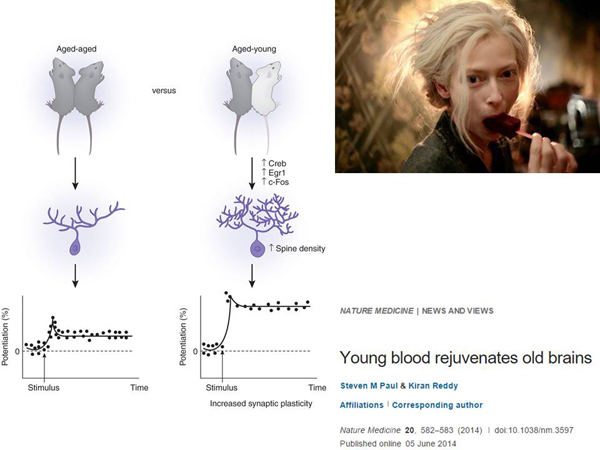
And the last one is life extension. In the fairy tale, the vampire girl was rejuvenated by drinking the blood of people. But in life everything is not as simple as in a fairy tale. There is such a thing called parabiosis. They take two rodents and combine their circulatory systems. At the same time, the old rodent lives more, and the young one lives less. With parabiosis, the effect of rejuvenation of the nervous system occurs, which was unexpected for scientists. Nerve cells partially develop plasticity – this is when nerve cells have the ability to form new contacts with other cells.
And so the plasticity is partially restored, and the regeneration of muscle tissues improves with parabiosis. It is clear that no one offers to stitch people together. But if it works, it follows that there are some factors in the blood of a young animal - cells, substances. And if we find something that is associated with a rejuvenating effect, we can make some kind of drug out of it in the future.
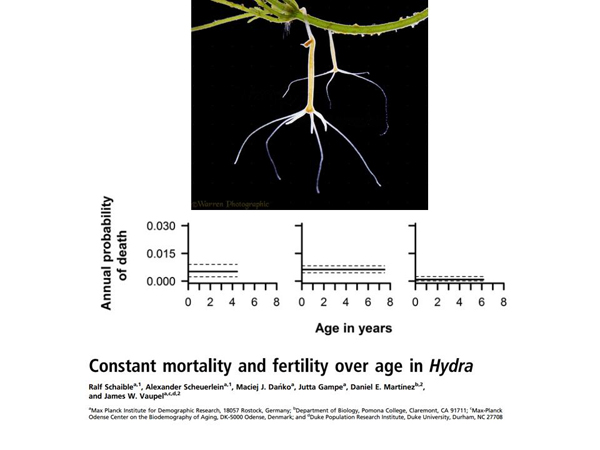
Another thing concerns the fundamental achievement of such a thing as negligible senescence. For us, for people, the probability of dying is growing every year of our lives. The probability of dying at 30 is significantly lower than the probability of dying at 50. These are hydra, whose probability of death does not change with age. These hydra were found somewhere in nature and put in a kind of individual incubator with comfortable conditions, where they have no threats to life, they live there and "do not deteriorate" with age.
Well, these are hydra organisms that are far from us. And closer?
For example, this naked digger. Normally, rodents live for two years. And these guys have been living for 25 years. This question is very interesting to scientists – why do they live so long? And they also very rarely get cancer, it used to be thought that they do not get sick at all. Why? Apparently, because they have an additional mechanism to control the growth of the tumor – the cells stop growing if there are too many of them.
Now these diggers are being actively studied to understand how they achieved such a long life, so that they can then apply it for us. But the fact is that the phenomenon of negligible aging has been detected for a mammal. This is not immortality, but the fact that it does not get worse with age.
And I would like to finish the lecture with the picture "The Triumph of Death". All over the world, not only in our country, a huge amount of money is spent on various nonsense – on the military budget, on the largest yacht, who has the largest yacht, the most expensive watches... It is clear that after some time - in 500 years – we will reach the technology of negligible aging, we will learn how to genetically modify people, make them better, and during their lifetime, and some bright future will come.
The question is whether we will live to see it. Okay, not us, so our children and grandchildren – will they live to see this bright future. And it depends on how we will treat science, how we will do it, and what role science will play in our economy in our country and in the world in general. That's why it seems to me so important to popularize science and fight for science to develop, and all sorts of obstacles to the wheels of scientific and technological progress were not put. This is one of the things that motivated me to write my book.
Before we get to the questions, I will mention the Evolution Foundation, which is trying to develop the idea of popularizing science by translating books from English into Russian, there is a website, you can go there and see. And now we turn to the questions, for the best question I will give a copy of my book. (Applause).
Discussion of the lecture
Boris Dolgin: Thank you very much. Let's move on to the questions, and according to tradition, I'll start with my own. Where memory was mentioned, why is it interpreted as memory and not as a normal conditioned reflex?
Alexander Panchin: This rodent was not electrocuted in this room – what was he supposed to have a conditioned reflex for?
Boris Dolgin: On the factor that causes him to panic.
Alexander Panchin: And what factor causes him to panic?
Boris Dolgin: Light.
Alexander Panchin: But it wasn't lit in that room.
Boris Dolgin: It doesn't matter where. If we imagine Pavlov's classic experiment with transfer to another room, then it would have to be interpreted as a reaction to interference with memory?
Alexander Panchin: No. There is all the necessary control. You can take this mouse into a room where it was not shone or electrocuted.
Boris Dolgin: But it will react to the light.
Alexander Panchin: It's not the room where she's being shone that she's afraid of. They don't expose her to any light in that room. She is brought into another room, where she is shone into the brain and electrocuted, but she is not afraid of the light, but of the room. Not light, but a room in which she was not subjected to anything. She was just in this room. In fact, I've simplified the description of the experiment a bit.
Boris Dolgin: I am interested in how it is proved that she is not afraid of the light, but of the room?
Alexander Panchin: There is no light in the place she is afraid of.
Boris Dolgin: Once again: what causes fear after what?
Alexander Panchin: The fear arises after the current and light were simultaneously given to her brain in another room.
Boris Dolgin: How is the fear of the first room checked?
Alexander Panchin: They put her in a room and watch how she behaves there. But they don't shine.
Question: The question of fifty shades of biotechnology. Actually, this is a fundamentally big difference and value – what kind of technology we are developing: increasing the length of the penis or whether we stay alive or not. At the end of the lecture there is such that "well, we will not live." Actually, it's the same in the book – "I think we probably won't have time." And then - 20 pages of text on how to do it. The whole complex of the anti-aging program. Nevertheless, why is there such a reservation "we won't have time" all the time? It is necessary to call society to the fact that there is a chance to make it!
Alexander Panchin: Everything is very correctly said now. When I write a book, I try to be as honest as possible. This is my assessment – I do not rule out that we can live up to these technologies. It would be great if we lived. The question is: how intensively will we do this? I am not ready to promise those present here that everyone will live. I say that we MAY live to see it. To increase the chances of "survival", it is necessary to make certain efforts. But I'm not ready to promise that this will happen. I'd like to live to see it, but I can't promise that.
Boris Dolgin: If I understand the meaning of the question correctly, one of its shades was whether it makes sense to design research so that there is something ahead that ensures survival and so on. Is this about this question?
Question (continued): This is a complex issue. Rather about the position: Alexander says that "the glass is half empty", and I suggest him to say that "the glass is half full".
Alexander Panchin: I have a very good attitude towards people who say that "the glass is half full", and I am very glad that there are such people, they make a significant contribution to the progress movement. But I'm just trying to demonstrate a certain level of honesty.
Boris Dolgin: That is, it is correctly denoted: what is already there, what will happen tomorrow, and what, in principle, can be, but it is impossible to name exact dates?
Alexander Panchin: Yes. So.
Question: Alexander, did you like the word you said: "honesty." Tell me, are scientists looking for the honesty gene? (Laughter in the audience).
Alexander Panchin: It should be understood that when they say the gene of something, it is often greatly simplified. Genes are usually not "something", but they do a lot of things. About honesty. I don't know any studies specifically about honesty, but by analogy: when looking for a genetic component to a propensity for violence, for example. They take people who have a history of violence and take those who do not have such a tendency.
They take a lot of them and look at whether there are variants of genes that are involved in the work of the nervous system, for example, that would be overrepresented in those people who differ in certain behavior. Some variants of these genes are found, and journalists quickly declare them genes of something, for example, the "gene of violence". This suggests that some features of our behavior are determined by genes. It cannot be said that any one gene is responsible for this, but a set of genes and some variants and their interaction with each other are associated with a particular form of behavior.
Question: I have a question about GMOs: there is a powerful debate going on all over the world, and there are still two camps. Some say that it is very harmful, others say that it does not affect the quality of our lives. What do scientists say about this?
Alexander Panchin: The topic of GMOs is a topic on which society – scientists, specialists and ordinary people – are as disjointed as possible in their assessments. There is the Association of scientists AAAS, the largest association of scientists in the world, which publishes the journal "Science". They were interviewed and 88% said that GMOs in products are safe. These are scientists of different profiles. It is clear that if you interview molecular biologists, there will be generally 90%. In Russia, 80% of the population believe that GMOs are harmful and should be banned, in America, about 70% of the population believe that GMOs are harmful. Why are people afraid of GMOs?
Question (continued): They believe that GMOs will affect the quality of life.
Alexander Panchin: They just don't understand what it is a little bit. A survey was conducted in the USA: do I need to label products containing GMOs? 82% answered that it is necessary to label. In the same questionnaire, to the question "Is it necessary to label products containing DNA?" 80% answered that it is necessary to label. If you ask how GMO stands for, then there are few who will answer.
Question (continued): I have a question about the newfangled CRISPR/Cas9 protein: how do scientists program it so that it cuts the molecule exactly in the right place for us, and how does it not make mistakes so as not to damage the body?
Alexander Panchin: CRISPR/Cas9 affects the DNA sequence that is complementary to the guiding RCN. This RNA, which is needed for the CRISPR/Cas9 protein to work, consists of two parts: there is a universal part and it is necessary for CRISPR/Cas9 to "understand" that this is the guiding RNA that it needs to look for something with it. He won't take any RNA and use it.
And the second part is the one that actually directs, it should be complementary (complementary) to some part that you want to cut. We create this RNA, we synthesize it. You can make a gene that will produce it in the cell itself. You embed a gene into the cell that will synthesize RNA and another gene that is needed for the synthesis of Cas9. Then a protein and the "right" guiding RNA are formed in the cell. And then this protein takes this "right" guiding RNA and uses it to look for something that is so complementary.
Question (continued): I also have not even a question, but a wish, which for some reason is being pushed into the background by scientists, although, it seems to me, it is promising - I'm talking about aging: we need to study people who have lived to 100-120 years, to 140 ... We need to study their genome…
Alexander Panchin: Up to 140 – there are no such. They study the genome, they study it. The maximum age is 122 years. Longevity record. In fact, they are studying what may be associated with super longevity. There is nothing to brag about yet. There is also a problem – since there are a lot of people, it may be that some of those who have lived to an extremely long age are random fluctuations. Roughly speaking, most people die, but some are lucky. There are hereditary components, of course. But to understand why centenarians live so long is, of course, difficult. But research is underway.
Question: I have a question for you, as a popularizer of science: don't you think that achieving immortality demotivates a person to develop? There's no hurry.
Alexander Panchin: It seems to me, on the contrary, motivates. My personal philosophical position is that, on the contrary, mortality devalues development quite a lot: you live, you live, you do something, and then you die once. It is clear that when you are not afraid to die prematurely, thinking changes, but I don't think it demotivates. It definitely wouldn't demotivate me.
Question: Tell me, as I understand it: CRISPR/Cas9 is a new cutting technology in the right place? Then how was it built in before this technology? Messy?
Alexander Panchin: In fact, life is so arranged that organisms like to embed something in themselves. The same bacteria can take a piece of DNA from the environment and embed it in themselves. When it came to genetically modified plants, we used such agrobacteria, which have a mechanism for transferring and embedding their DNA into the DNA of other plant cells.
They have a bunch of proteins that do this. There was such a technology: they took a bacterium and "said" that now, a bacterium, you will transfer not your genes, but ours. And the bacterium did it for us. There are viruses that can embed, but they embed somewhere. The peculiarity of this thing is that we say where it is necessary to make an incision in order to embed it there more likely. A lot of technologies are being developed now. This is the most "pop", there are other mechanisms of precise cutting, other approaches. They are also developing, but not as popular, because they are not heard.
Question (continued): And the second question: about the ethical side. When there is a question of biotechnical developments of an anti–aging mechanism, a long life is on one side of the scale, and on the other - the problem of overpopulation, resources, and the prospect of such technologies is even frightening. Who decides?
Alexander Panchin: I have a bad idea who decides this and how. In fact, the idea that the fight against aging will lead to demographic problems seems to me a little far–fetched - if we look, we will see that problems with overpopulation arise in countries with poor living standards and this is due to the fact that people think differently.
If you assume that you will become old and infirm, the state will not pay you, then your only hope for a normal old age is a lot of children, with a margin. Because some of them will die from malaria, and some of them will die from something else. You can hope that one of them will not turn out to be a reptile and will feed you and take care of you. This idea is in the culture of many countries.
And when you achieve high scientific and technological progress, when people rarely die, when you have high labor productivity, and one person can feed a hundred, and agriculture is effectively organized, then everything changes for you. You no longer need to worry that you will not have descendants who will take care of you. And we see that in the most developed countries there is a demographic decline. It is a sign that you are doing well.
Boris Dolgin: I should note one more thing: so far, history shows that attempts to stop certain technologies turn out to be dead ends. You can stop in one place, the technology will "come out" in another. They can only be slowed down a little. But in general, this will not change the situation.
Alexander Panchin: I will supplement it. Imagine if everyone said they wouldn't make genetically modified embryos, and China said they would. And any person who has enough money will just go to China and use this technology there. And as a result, China is developing, and other countries are starting to grab their heads and say that they have lagged behind scientific and technological progress.
Question: The example of a digger is not entirely clear. Isn't he getting old?
Boris Dolgin: I will explain the question: why did you give a naked digger as an example of unusual longevity, if there are organisms that live much longer than 25 years?
Alexander Panchin: I understood. It is given as an example because it is an abnormally long-lived organism for a mammal of this size. A tree in this sense is not very interesting, it differs from us in millions of indicators, and making a person more like a tree is not a very constructive way. And here we see that there is one rodent and another, a rat and a digger, very similar to each other.
But for some reason, the digger lives much longer – it means that he has had some mechanism for quite recently that helps him to live for so long. And if we find this mechanism, we have a higher chance of applying it to other mammals, in particular to us. And the tree, of course, lives for a long time, but it doesn't really apply to us. And bacteria – they, of course, divide in half, but it doesn't help us either.
Boris Dolgin: In other words, if there was a species closer to a person, more recently departed from this branch, but with an unusual longevity, would it be investigated? And so the closest centenarian is a naked digger?
Alexander Panchin: Yes.
Question: At one time, regarding the randomness of evolution, Wolfgang Pauli (Wolfgang Ernst Pauli) came up with the following argument: that if evolution went randomly, changes went in an arbitrary way, then the observed length of evolution would be much longer.
Boris Dolgin: And why all of a sudden?
Question (continued): Randomness is randomness. While all options will be sorted out…
Boris Dolgin: "Until all options are sorted out" – and then?
Question (continued): Until all the options are sorted out, they will survive, they will not survive until they produce meaningful offspring… As if you give a monkey a typewriter, then with some probability it will print "War and Peace". That is, are there any mechanisms of directed evolution or something that would explain all this?
Alexander Panchin: I understood the question. The example about a typewriter is very good to show misconceptions about how evolution happens, which are generally very common. The idea that the monkey printed "War and Peace" is wrong. In fact, this is not how it happens.
You have conditional monkeys typing some meaningless text. But sometimes, for some reason, a word turns out there. And this word is better than nonsense. And a phrase in which there is a word, for example, "Masha", is much better than the one in which there is no word "Masha". Monkeys leave the word "Masha" and then print something. Suddenly it turns out "Masha sat down" or "Masha ate". This is already better than just "Masha". And "Masha ate a candy" is even better! And so at each stage there is something that was better than the previous one.
Boris Dolgin: I apologize. Is this a mechanism that will encourage this, and need a self–learning system mechanism?
Alexander Panchin: Yes. There is a video on this topic on Youtube called "The Blind Watchmaker". The famous "if you shake a broken watch in a drawer, the watch will not come together." That's right, because evolution concerns those objects that have the ability to reproduce, which have selection – a certain criterion when someone survives and someone does not survive, and there is hereditary information.
And the man made a computer simulation: the clock breaks, but then these laws begin to take effect. The clock can be crossed according to some rules, to get offspring, from which the one that will show the time better is selected. There is even a kind of evolution: first, a pendulum clock is obtained, then – with arrows, then – a three-shot clock. In general, this is a more correct idea of evolution, what happens and how.
Question (continued): In this case, next: what determines meaningfulness?
Alexander Panchin: In this case, nothing.
Question (continued): That is, external conditions determine survival in a given environment?
Alexander Panchin: Yes.
Question (continued): Well, we can so easily slide to Lysenkoism. That by setting the right conditions, we will get the right result.
Alexander Panchin: And so it happens! I was talking about scientists who received red fluorescent proteins. They wanted to get red fluorescent proteins. They set conditions, saying: "We have a random mutation, but if, as a result of the mutation, the protein turns from green to slightly redder, then we encourage these bacteria, multiply. And as a result, by random mutations and such selection, they turned out red fluorescent proteins. And our scientists are replaced by the environment. If for some reason in the environment it would turn out that red protein is better than green, then red would turn out.
Question: As I noticed, all these mechanisms in biotechnology revolve around the same thing – molecular scissors, containers, vectors. Can you predict – what else can you do in principle? On the one hand – as toys, on the other hand – for use. As for GMOs, of course, they are safe, but the political and economic situation has developed in such a way that it is undesirable to clone them now. And yet – the older a person is, the more difficult it is to study him, his tissues.
Alexander Panchin: The question was about what else can you come up with? If you really delve into fiction, it seems to me that in the future there will be such small nano-robots editing DNA using some "cool" mechanisms, patching up all the "holes" of the body, in every cell.
I think that genetically modified human cells will definitely be used, in a very broad sense, which will replenish the pool of our cells with special, more "cool" cells. We will not modify, for example, skin cells. And we will introduce stem cells that will gradually replace existing cells with new, modified ones.
There will be versions of a person – "version 2.0", for example. They will say: "Oh, you're so sluggish! Are you probably still on version 3.0? Come on, put, like me, version 4.0, you will run faster, the girls will like it more!" We will "upgrade" ourselves – I injected myself with something and ran. Like Lemma – when people learned to "dirty themselves", different subcultures began to arise: someone considered it necessary to simplify, reduce ears, remove five fingers and leave only three. And someone thought that it was necessary to become more complicated – to increase the number of limbs, teeth. It's better to read science fiction on this topic, of course.
And from what is being done now: one of the "cool" things is nano-boxes. We can make such boxes, inside of which you can stuff something, like the same genes. And the boxes themselves can consist of the same DNA or something else, and they will open when they come to the right cell and inject something there. These are the "cool" technologies of all kinds now, they will also someday find a "cool" application.
Question: The question is: if we ever find some elixir of eternal youth? What does "the elixir of eternal youth" mean – that is, more and more new cells will do something for us. But let's say it was built into a person. He got the "immortality gene" and some mutations, for example, a predisposition to cancer. It turns out that he divides a lot of cells and at the same time there is still a predisposition to cancer. Wouldn't that be a paradox?
Boris Dolgin: Perhaps I would expand on another question. As life expectancy increases, we are faced with those diseases that people, living to the age of 30, have not encountered. Accordingly, there is a need for new medicine and so on. How much do you expect new medical problems after the life expectancy changes significantly? I'm not talking about eternity yet. Was it about this? No?
Question (continued): I'm talking about the fact that a person reproduces, transfers his genes to other people, and it turns out that the gene of immortality – let's say he wanted to reproduce…
Boris Dolgin: And, your question is just whether, when developing a tool that will be found for such an infinite lifespan, it will be taken into account when developing it that it can somehow be modified as further reproduction proceeds?
Question (continued): No, don't modify!
Boris Dolgin: Wrong again?
Remark: I understand the question. The author of the question is still slightly confused and considers the "immortal man" in generations – immortality, so immortality. And here, as far as I understood the question, is there a contradiction: a man lives, he has cancer here, and he, in theory, should die of cancer. But cancer is only a disease. And the same naked digger does not have cancer. And a means that prolongs life, and even more so – achieving immortality, is a means that automatically prevents oncological diseases. Otherwise we have no "elixir of immortality".
Alexander Panchin: In a sense, yes.
Question: What is the budget of biotechnologies? What can be cheaper there so that the technology comes to a sufficiently household level – in schools, in institutes?
Alexander Panchin: See. In fact, simple experiments on genetic engineering are already being done in Western schools. It's not very expensive. If we take fundamental science, then people with RFBR grants – about 500 thousand rubles a year for a laboratory – manage to do research on genetic engineering in laboratories. Someone gets plants with a human gene or a bacterium with a human gene in the laboratory. I did this when I was a student.
Yes, we need some equipment. Besides, it's not very expensive. What is expensive? That you have done something, and now you need, firstly, it to be useful, and then - to have it honed and passed through all regulatory authorities. If you want to make, for example, a genetically modified plant - for example, wheat growing on salty soil – it is not very difficult and not very expensive. But before entering the market, you will be told that you need to pass 1500 tests, something else…
Question (continued): That is, the most expensive thing is the fight against bureaucracy?
Alexander Panchin: If we are talking about the commercial implementation of the project, then this is a huge test for security and the fight against bureaucracy. These things are a bit related, because now there is an opinion, in particular – and mine too – that we are overly concerned about safety when it comes to GMOs. And we poison all kinds of mice in vain. We know that it shouldn't be more dangerous than a breeding variety, and we don't subject breeding varieties of plants to such checks, do we? Somehow you need to expose, because everything can be – by random mutations, potatoes rich in solanine can turn out. It is clear that the quality control of products should be at the state level. But all the tests to check the safety of GMO organisms is that we already know that there is no difference. And it takes a lot of money.
Boris Dolgin: In other words, is it a question of finding the optimal level of security guarantee and the optimal price?
Alexander Panchin: This is a matter of risk management. There are different assessments of the riskiness of the technology. Depending on what your risk assessment is, you spend different amounts of money to check whether these risks have been realized or not. And the problem is that now the risk assessment is too high compared to what it should be adequate. Most likely, we need a scientific discussion about an adequate assessment of these risks.
Boris Dolgin: Well, yes. But then it is displayed in the public field, because the decision is made everywhere (public).
Question: Your prognosis for gene therapy: after what period of time will it enter into everyday practice and it will be possible to use it, for example, in a clinic in America, in Russia?
Alexander Panchin: I think it's a matter of a decade. Look: there is already gene therapy, it is available to very select people. Moreover, not that very rich people, but those who can personally fall into the list of people who will be tested, roughly speaking. But there are already companies that have almost brought the product to market. It costs about a million dollars.
Question (continued): That is, the question is the price?
Alexander Panchin: Yes, the question is the price.
Question: Tell me, are there any developments on the treatment of Down syndrome using genetically modified cells?
Alexander Panchin: To be honest, I haven't heard. I think there should be more prevention in terms of perinatal diagnostics – an effective way of prevention. But when this syndrome has already arisen, a lot has already gone wrong. Even if we remove this "extra" chromosome from all cells, then those tissues that have already formed incorrectly, they will no longer disband, will not become "correct". I don't think there will be a gene therapy for Down syndrome in the near future.
Question: Good evening. The question of viral delivery of gene material into cells. The virus is very small, tens or hundreds of nanometers – how to insert the gene material into the capsid (the outer shell of the virus)?
Alexander Panchin: The virus has a certain fixed length size, there are certain areas specific to this virus that are responsible for "packing" into the capsid. And thanks to this, the capsid, which is synthesized inside the cell, recognizes what it has to put in itself, puts it in and a whole viral particle is formed.
The principle is that you will find out through research what exactly in the viral DNA or RNA molecule is responsible for "packing" into the capsid, what limits, the size of this sequence, then you try to cram your genetic construct so that it satisfies these sizes and all these conditions. And after that, somewhere in one place you produce a capsid, in another place you produce your construct.
From here you take only the protein fraction, if the capsid is protein, so that the viral hereditary information, the original that this virus had, does not get there. And from here you take only hereditary information, you mix it all in a test tube and select some specific conditions for this "assembly" to take place. After that, you freeze so that the "structure" does not die and transport it.
Question (continued): How can this be done if a virus with a minus "p", which, among other things, needs its own viral polymerase?
Alexander Panchin: Firstly, not all viruses are used. Different viruses have different qualities for gene therapy. And, secondly, you can transfer some viral genes in some cases. If you want to use, for example, a retrovirus, then it is clear that you need its reverse transcriptase, which embeds genes into DNA, because that is what you want to do, but you can mess up other genes so that the virus does not reproduce after all.
Question (continued): You mentioned some universal immune cells that can be given to any people, and they will not be attacked by the already existing immune system. How do they get them, how do they deal with histo-compatibility?
Alexander Panchin: To be honest, I'm afraid to lie now, I don't know all the details of the process. They have come up with something that allows these cells to avoid detection by the immune system. How it's done – I'm afraid to lie. A very good question, very correct, but this is already a narrow area where I haven't dug deep enough yet.
Question: The question is about evolution. Based on your lecture, I realized that mutation can occur due to bacteria that embed something in DNA, viruses, maybe radiation. Do these mutations always occur under the influence of external factors? Is the condition necessarily the impact of external factors?
Alexander Panchin: See. Mutation always happens. Even the very process of doubling the DNA molecule, when you want to get two cells from one cell, the DNA must first double. Even in this process, mutations can occur. The enzyme that doubles the DNA molecule, it's not perfect, sometimes it makes mistakes.
On the other hand, what happens from this side in bacteria, what else is this thing interesting from the point of view of evolution – in fact, this is the first and only example of Lamarckian evolution shown in the world. There is evolution with random mutation and natural selection, here the mutation is not random, a certain piece of the virus was embedded somewhere there. And it is inherited.
This is such a curious example, but then Darwin's evolution takes place, because sometimes it is built in incorrectly, from this the bacterium dies. And in general, many bacteria do not survive a viral infection.
Question: During the lecture, you mentioned the plasticity of the brain. I read in the scientific literature that there is some kind of protein that can "turn off" a gene. Is there any such technology?
Alexander Panchin: "Turning off" genes is easy enough. It's funny enough how it's described: "What needs to be done to turn off the gene? You have to make a copy of this gene, reversed backwards." It can certainly be funny here – (if) a literary metaphor is inserted. We eukaryotes – plants, fungi, animals – have such a mechanism called RNA interference.
Our cells don't like double-stranded RNA, all our RNAs are from the same chain. Although, in principle, RNA could be double-stranded, like DNA. Because so many viruses use double-stranded RNA as a carrier of their hereditary information. And when our cell recognizes double-stranded RNA, it immediately says "Oh, this is some kind of nastiness!", takes a piece of double-stranded RNA, makes a single-stranded one out of it and goes to look for all similar ones and cut them into pieces.
And this leads to the effective "shutdown" of any gene that is similar in sequence to this RNA. Therefore, if you have a normal gene, you inserted a copy of this gene into the body, inverted backwards, you form a double-stranded RNA, and both of these genes are "cut down", in fact. We always know what needs to be done to turn off the gene.
Question (continued): Is it possible to treat something, some diseases in this way?
Alexander Panchin: There are diseases associated with the fact that some gene is too active, and we can crush it.
Question: Biotechnologies are a kind of production. Is there any production plan? What do commercial enterprises do?
Alexander Panchin: I have only been to scientific laboratories where they are engaged in production. In fact, it's very similar. The peculiarity of bio-production is that you have made a good plant variety in the laboratory, given it to the farmer, he has everything grown up. And then it reproduces itself. Therefore, production in this sense is quite simple.
There is a separate area – biotechnological production of medicines with the help of microorganisms, that's right: there is some kind of tank, a reactor where genetically modified organisms are planted, they are fed in large quantities with nutrients, they produce something there, then something is taken from there, purified and some kind of finished drug is obtained and so on. But when it comes to the creation of genetically modified organisms, then it's like in breeding - you have done, received a new variety and continue to propagate it.
Question (continued): But the cost of reagents? For example, how much can CRISPR/Cas9 cost?
Alexander Panchin: Depending on what you want to do. The question is very complicated. If you already know what kind of mutation and where exactly you want to do it, in an organism that is a model – people already know how to grow it, know how to work with it. If you ask to genetically modify, for example, a panda that does not want to breed in captivity, then you will have problems. But if you want to genetically modify tobacco, which in any laboratory of plant physiology can be used as a model organism, then everything is easy and cheap for you. Because there are already people who know how to grow it. It all comes down to such details.
Boris Dolgin: If I understand correctly, are you interested in the last link in the chain "science-technology-entrepreneurship"? Maybe we'll have that one day, too.
Question: Tell me, how do scientists "copy-paste" genes into the genome of bacteria? I am engaged in microbiology: from a test tube to a flask, from a test tube to a flask. How do geneticists do this?
Alexander Panchin: When I was doing this during the course work, it was a long time ago, since then everything has changed. We had frozen bacteria that had certain genes in them. We had samples of plasmids with certain genes frozen. That is, there is already a ready-made ring DNA, where there is something, and we know how these plasmids are arranged and therefore we can cut out of it.
We can bring this plasmid into a bacterium, multiply the bacterium, then isolate the DNA, get this plasmid and freeze it again. Thus, to replenish the amount of material with which you can work. There was an approach that we could order from some company, and they synthesized some kind of DNA molecule with a certain sequence on a chemical synthesizer, and we could embed it somewhere, or they could embed it themselves into some kind of plasmid.
Question (continued): How does embedding work?
Alexander Panchin: We know exactly the sequence of this plasmid and which nucleotides sit where. We want to cut out a piece of this plasmid, we select two pairs of scissors so that one cuts on the left, the other cuts on the right, then we separate a small fragment of what we cut. After that, we mix it with something else where we want to insert it.
If we want to transfer a piece from one plasmid to another plasmid, we cut one plasmid, select an insert, take another plasmid, cut it and add an insert there, and then add an enzyme that stitches back together. There are enzymes that were invented by nature: there are enzymes that are cut in certain places, that stitch certain types of incisions, and we can do all this in a test tube.
Question (continued): At the very beginning of the lecture, you mentioned fluorescent microorganisms, and you said that they could be used to find the pathways of neuronal connections. What other practical use do these fluorescent microorganisms have besides paintings? Where else can this technology be used to produce fluorescent organisms?
Alexander Panchin: The most important field of application is fundamental science, because one of the main tasks for us is to understand what a particular gene does, where it works, and so on. And here this technology is absolutely irreplaceable. Here its most important application. And then other technologies follow, which are already further used in other areas. For example, they came up with the idea that you can make a clock based on fluorescent proteins.
There are such fluorescent proteins that fade over time and you can make such a cell, a fluorescent protein is produced in it. If some kind of reaction is triggered, first it is green, and then it dims. And you can see how long ago the impact was made on the cell of the type that interests you. It is possible to make detectors in this way – it is possible to make such a system that, if, for example, a certain substance is present in drinking water, then the microorganism living there begins to produce a fluorescent protein. Here is such an application, for example.
Question: Tell me, isn't there such a danger that when we change the protein marker that HIV reacts to, then after some time, when we change it in a certain percentage of people, HIV will also mutate, and we will get a strain that is able to infect two types of cells? And we can't change the protein indefinitely, because sooner or later it will break, and we need it for some reason.
Alexander Panchin: Yes, we need protein for some reason, yes, and we even know why. People who have this protein completely spoiled have side effects: they can even be fatal in rare cases. When you have a specific person with HIV, you want to cure him – this is an option to help a specific patient. In the future, some other virus will appear that will have a different mechanism.
We cannot take and defeat all the viruses of the future that may arise in one fell swoop. It is clear that in the future we will have to come up with new mechanisms against new viruses. What is good about the option using CRISP/Cas9 – we can make immune cells from any virus. At least, from those that we now know. A new virus appeared – Cas9 was updated into the system – and that's it, stability appeared. But for now it's such a fantasy, which in the future, maybe, will become a reality.
Question: We have already asked about the treatment of Down syndrome. And if the embryo was diagnosed at an early stage with some other mutation, not genomic scale, but chromosomal or genetic? Is it possible in this case to use bio-technological methods of treatment?
Alexander Panchin: Basically, yes. This is why experiments on the genetic modification of human embryos are needed. Another question is what it could be, like firing a cannon at sparrows. Why? Almost always, if we are talking about a genetic mutation in an embryo, it is easier to choose a healthy embryo.
All families are recommended to undergo a genetic analysis for the presence of known genetic diseases before having children. After that, if both parents are carriers of some dangerous genetic disease, then they are recommended to do artificial insemination.
And at the same time, we can guarantee that the embryo that will be implanted to the mother will not have the disease that threatens the unborn child. This is more expedient than climbing into the embryo with the help of genetic engineering and changing something there. But, in principle, if someone wanted to do this, the technology allows it. But it has not yet been worked out on human embryos. There are always different pitfalls.
Question (continued): In nature, in isolated reservoirs or in remote territories, there are organisms that die from natural causes – predators, diseases. They found some very old birds, fish. Do all organisms age?
Alexander Panchin: I have given two examples of organisms that do not age.
Replica: You're saying everything right. Isolation, the absence of predators and problems in animals moves evolutionary selection to increase life expectancy. The same naked digger – he lives in huge burrows and is protected from predators, just like the "Brandt's Moth" – this bat has been living for 42 years and is even more interesting than the digger.
Alexander Panchin: Now I understand the question! and the answer. (Laughter).
Question (continued): Does eusociality have any effect?
Replica: Here we can assume that eusociality is a favorable factor for the animal, the same as size. It's hard to devour a large organism. And, accordingly, it is better protected. In fact, only three groups have been studying the digger for the last few years, and we know very little about them, including about the causes of death.
Alexander Panchin: If it is true that only the female lives for a long time, then this is even more interesting, because comparing the female with other organisms is even more productive in terms of search. But I don't know for sure, to be honest.
Replica: There the female brings offspring with dominant males, there are 3-4 of them. Quite often. Dominant males, apparently, also live a long time.
Question: Genes are genetic evolution, we see the diversity of life on Earth. Memes are the evolution of knowledge, we see the diversity of knowledge on Earth. And will there be some kind of mega-evolutionary step further?
Boris Dolgin: I would make an amendment that if genes are an established scientific category, then memes are still a kind of journalistic metaphor.
Alexander Panchin: And I will not agree. Memes exist, they are given to us in empirical observations. Like any idea, they undergo mutations and have all the conditions necessary for us to consider an object evolving. They have descendants, descendants may differ. There is selection and so on.
Boris Dolgin: This is a metaphor that you can try to work with, but it has not acquired a terminological status.
Alexander Panchin: The idea is that the features of our brain work depend on which ideas seem more attractive to us. Why do we like poems more, let's say, than the same idea expressed not in verse? Why does rhyme give a certain appeal to the text?
These are the kind of things – the features of the structure of our psyche that affect what kind of forms and what kind of ideas are distributed more effectively in our population among people. This is very interesting, there are experimental works that are devoted to comparing: how changes in some idea affect what people remember well and give this idea further to other people. This is an experimental study of mutation in memes. But not a lot of people do it.
Stanislav Lemm had a super-fantastic, scary thing where people arrive on some super-perfect planet, which is a single crystal. He knows how to react correctly to any external influence, comprehended all the laws of physics and achieved absolute perfection. Therefore, it is not known where everything can go, maybe some other virtual reality will be formed in which it will be possible to exist perfectly while being completely safe. Underground, in a bunker.
Boris Dolgin: Judging by the way the question was asked, we are talking about whether you predict the appearance in some other area, in some plane, of some type of unit similar to genes and memes, with which it would make sense to work to study some other form of evolution?
Alexander Panchin: Now a complete nonsense has come into my head, for example, the evolution of corporations. Huge communities of people. Just as a person is made up of cells, a corporation is made up of people. And this group of people can "eat" another group of people. Or multiply by creating a franchise, for example.
Boris Dolgin: Strictly speaking, some attempts to apply the theory of the evolution of society belong to the XIX century, and not that it was very new for the XXI century. Thank you for the lecture!
Alexander Panchin: I was asked to donate a book for the best question. It seems to me that this girl asked the best question that I didn't know the answer to. So the book is hers.
Portal "Eternal
youth" http://vechnayamolodost.ru 14.06.2016